Research Excellence: Metallic Bloom
Graduate College
Mentor: Vivian Merk, Ph.D.
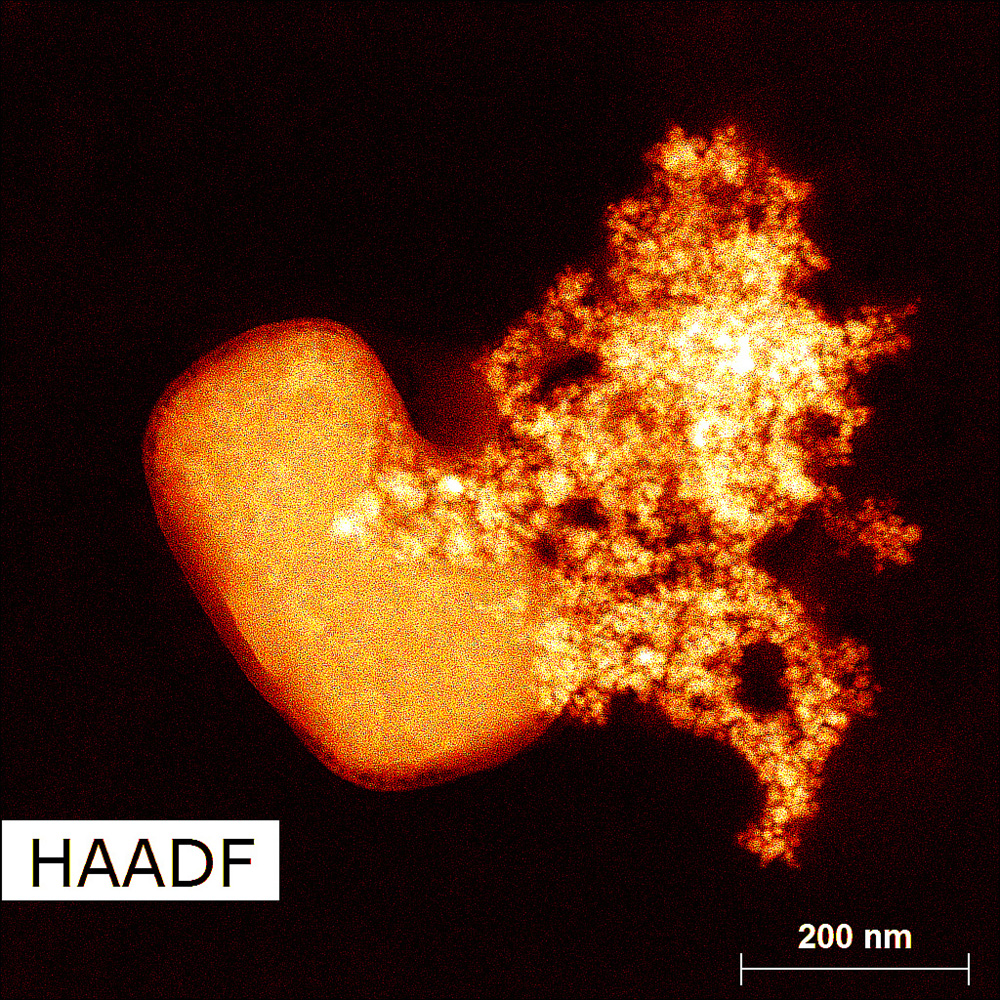
This image was captured using specialized imaging called High-Angle Annular Dark Field with a specific type of microscope called scanning transmission electron microscopy. It uses electrons instead of light to reveal structures far smaller than what regular microscopes can detect. The bright cluster shows nickel particles formed by heating chemicals to 170°C in a polyol liquid called ethylene glycol. The polyol acts like a chemical cooking medium, helping control how the nickel particles form and grow. Though the particles didn’t grow on the salt crystal, the surface beneath them looks like one, creating the illusion of a metallic bloom. Each particle is merely a billionth of a meter wide, several times smaller than a grain of sand, yet together they form a branching pattern shaped by heat and chemistry. Collaboration with Brookhaven National Laboratory