Elucidating Molecular Circuits Supporting Neuronal Signaling and Health
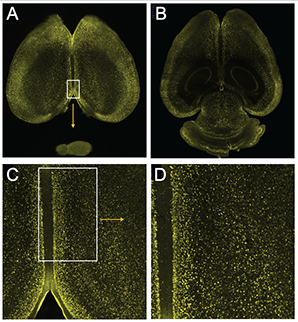
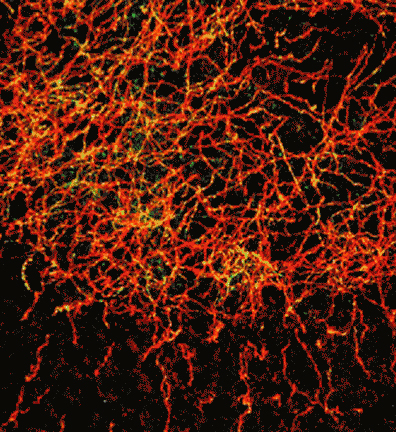
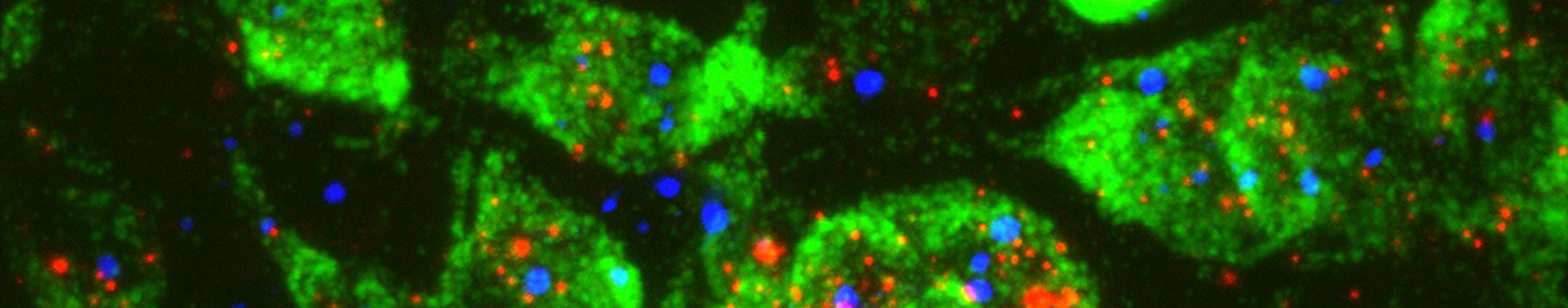
Optical sectioning of a whole mouse brain via the Blaze Light Sheet Ultramicroscope demonstrates c-fos labeling at increasing magnification moving from A to D where individual activated cells are readily detected.
c-fos labelling showing neuronal activation on a whole mouse brain. After immunolabelling and tissue clearing, brain was scanned on the Blaze Light Sheet Ultramicroscope. Credits: Lorena Areal, Paula Kurdziel and Jana Strickler.
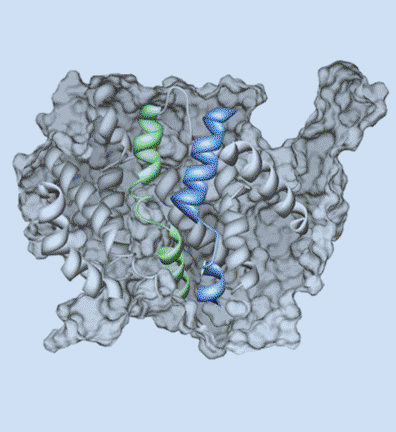
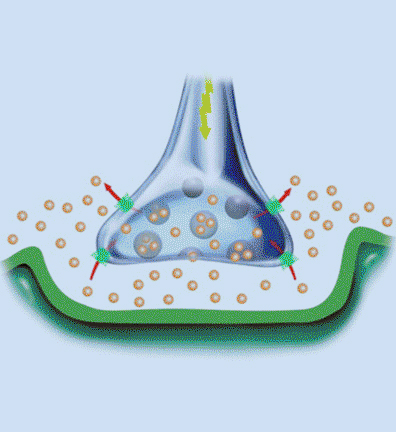
Research in the Blakely laboratory focuses on the structure, regulation and disease relevance of neurotransmitter transporters, proteins that govern the inactivation of chemical signals released at synapses to elicit target activation and inhibition and ultimately control the way we think, move and behave. The work utilizes a range of physiological, optical, genetic and genomic methods to understand how changes in transporter function can support neuroplasticity as well as neural disorders. The researchers use multiple model systems that include the powerful genetic model C. elegans, where discovery of molecules that support neural signaling and health is enhanced by rapid growth and ease of genetic manipulation. Other studies utilize genetically modified mice whose complex circuitry and behaviors align with those present in humans and can allow for modeling of neurobehavioral and neurodegenerative disorders. In collaboration, the researchers pursue studies in humans exhibiting the molecular and circuit changes they have observed in animal models and aid in the development of novel therapeutics. Together, these tools, models and paradigms are pursued to advance understanding, diagnosis and treatment of brain diseases that impact the lives of millions worldwide, disorders ranging from ADHD and autism to neuropsychiatric disorders and neurodegenerative diseases such as Alzheimer's and Parkinson's disease.


Kappa opioid receptor antagonism restores phosphorylation, trafficking and behavior induced by a disease-associated dopamine transporter variant.
Mayer FP, Stewart A, Varman DR, Moritz AE, Foster JD, Owens AW, Areal LB, Gowrishankar R, Velez M, Wickham K, Phelps H, Katamish R, Rabil M, Jayanthi LD, Vaughan RA, Daws LC, Blakely RD, Ramamoorthy S.
Mol Psychiatry. 2025 May 29. doi: 10.1038/s41380-025-03055-4. Online ahead of print.
PMID: 40442453
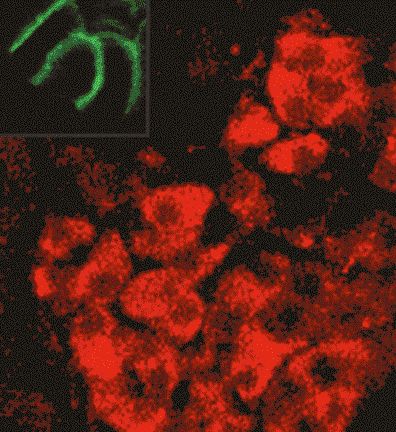
Glial swip-10 controls systemic mitochondrial function, oxidative stress, and neuronal viability via copper ion homeostasis.
Rodriguez P, Kalia V, Fenollar-Ferrer C, Gibson CL, Gichi Z, Rajoo A, Matier CD, Pezacki AT, Xiao T, Carvelli L, Chang CJ, Miller GW, Khamoui AV, Boerner J, Blakely RD.
Proc Natl Acad Sci U S A. 2024 Sep 24;121(39):e2320611121. doi: 10.1073/pnas.2320611121. Epub 2024 Sep 17.
PMID: 39288174
Forward genetic screen of the C. elegans million mutation library reveals essential, cell-autonomous contributions of BBSome proteins to dopamine signaling.
Refai O, Rodriguez P, Gichi Z, Blakely RD.
J Neurochem. 2024 Sep;168(9):2073-2091. doi: 10.1111/jnc.16188. Epub 2024 Aug 8.
PMID: 39118406
Novel anti-inflammatory effects of the IL-1 receptor in kidney myeloid cells following ischemic AKI.
Chen Y, Lu X, Whitney RL, Li Y, Robson MJ, Blakely RD, Chi JT, Crowley SD, Privratsky JR.
Front Mol Biosci. 2024 Apr 17;11:1366259. doi: 10.3389/fmolb.2024.1366259. eCollection 2024.
PMID: 38693918
Long COVID-19 and Peripheral Serotonin: A Commentary and Reconsideration.
Anderson GM, Cook EH, Blakely RD, Sutcliffe JS, Veenstra-VanderWeele J.J
Inflamm Res. 2024 Apr 11;17:2169-2172. doi: 10.2147/JIR.S456000. eCollection 2024.
PMID: 38628604

Randy D. Blakely, Ph.D.
Executive Director
FAU Stiles-Nicholson Brain Institute
David J.S. Nicholson Distinguished Professor in Neuroscience,
Professor, Dept Biomedical Science
Charles E. Schmidt College of Medicine
Click here for Dr. Blakely’s Curriculum Vitae.
Click here for Dr. Blakely's professional biography.
Updated 1/2025