Novel Study Demonstrates Role of Parkin Gene in Eye Lens
A new study at FAU is the first to demonstrate that the Parkin gene is turned on when cells are exposed to environmental insults that cause free radical formation and cataract formation.
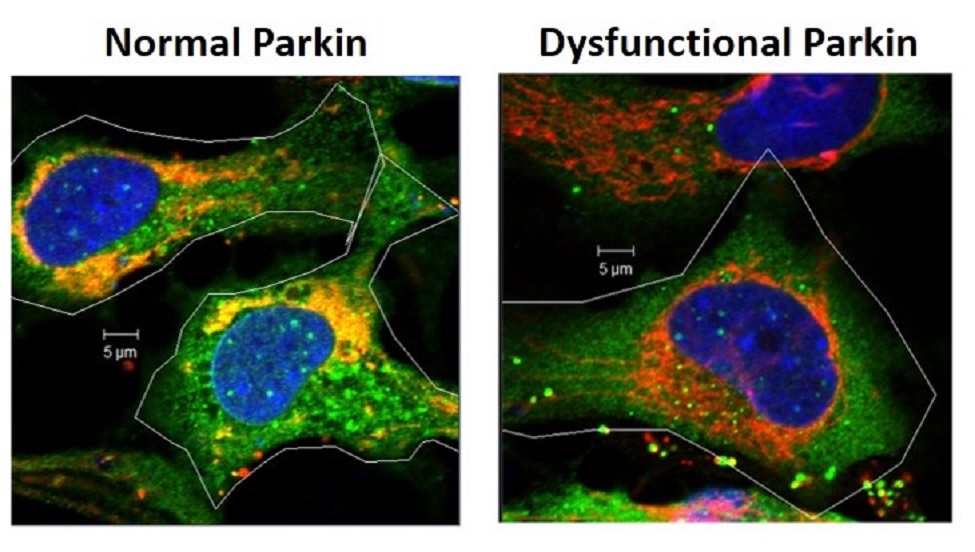
To discover new cellular functions for Parkin and how loss of Parkin function could contribute to age-related diseases, a team of researchers in FAU's Charles E. Schmidt College of Medicine engineered eye lens cells to make either normal Parkin or a mutant form of Parkin.
Although mutations in the PARK2 gene that encodes the Parkin protein are associated with developing early-onset Parkinson’s disease (PD), much remains to be discovered about how Parkin functions in cells and how loss of Parkin function could contribute to PD and other degenerative diseases. Parkin is known to regulate the degradation of mitochondria that are cellular organelles that generate energy required for a multitude of cellular functions. Damage to mitochondria is associated with the production of free radicals during the aging process and is involved in the development of multiple diseases ranging from neurodegenerative diseases to cataract formation.
To discover new cellular functions for Parkin and how loss of Parkin function could contribute to age-related diseases, a team of researchers in the Charles E. Schmidt College of Medicine at Florida Atlantic University engineered eye lens cells to make either normal Parkin or a mutant form of Parkin, and examined whether Parkin function was required for lens cell mitochondrial function, and lens cell survival.
Results of the study, recently published in the journal Biochemica et Biophysica Acta: Molecular Basis of Disease, are the first to demonstrate that the Parkin gene is turned on when cells are exposed to environmental insults that cause free radical formation and cataract formation. They also discovered that through the removal of mitochondria that are damaged by these environmental insults, Parkin prevents free radical formation in lens cells and increases the ability of the cells to survive exposure to conditions that are associated with aging and the development of many degenerative diseases. The data suggest an important function for Parkin in promoting the survival of not only lens cells, but of many cells of the body including neurons, and they suggest that activation of the Parkin gene could prevent cell damage that is associated with age-related cataract formation and death of neurons associated with PD and other age-related degenerative diseases.
“We found that Parkin is necessary to maintain lens cell function and that loss of Parkin function likely contributes to the formation of cataracts and possibly other age-related degenerative diseases that are associated with loss of mitochondrial function and increased free radical formation," said Marc Kantorow, Ph.D., senior-author, professor of biomedical science, and assistant dean of graduate programs in FAU’s Charles E. Schmidt College of Medicine. “Our findings suggest that Parkin plays a direct role in the prevention of oxidative stress through its ability to maintain cellular mitochondrial populations. Our data also suggests that the gene encoding Parkin is induced upon environmental damage so that drugs or genetic methods to increase Parkin levels and function could prove effective in the prevention of cataracts and other age-related degenerative diseases including neurological diseases like Parkinson disease.”
Kantorow and co-authors Lisa Ann Brennan, Ph.D., research associate professor in FAU’s College of Medicine, and Josef Khoury, a Master’s degree student in the Kantorow Laboratory, hypothesized that Parkin also may be important for the embryonic development of the eye lens since regulation of mitochondrial function is a key feature of the development of all tissues.
“We found that levels of Parkin based on genomic sequencing of eye lens at different growth stages exhibited different levels of Parkin suggesting that Parkin had region-specific functions in the eye lens,” said Brennan. “We were looking for genes that are changed during the normal growth and differentiation of lens cells to help identify those genes that control how undifferentiated stem-like cells become mature transparent eye lens cells.”
It is estimated that more than 6 million people in the United States suffer from some kind of degenerative neurological condition including PD. Despite advances in surgical techniques, cataracts remain a significant cause of world blindness. It is estimated that any therapy that could delay the onset of cataracts by just 10 years, could reduce the number of cataract surgeries annually by half, and moreover, improve the quality of visual health care while reducing costs.
“The next step of our ongoing research is to work to establish how Parkin regulates the growth and development of the eye lens by controlling mitochondrial populations that are required for lens cell growth,” said Kantorow. “We want to be able to identify the genetic mechanisms that regulate the production of Parkin in cells and to see if they can be manipulated to increase Parkin levels, thereby increasing cell survival to prevent disease.”
This work was supported by a $2.7 million National Institutes of Health (NIH) grant (EY02678) to Kantorow and his collaborators from the National Eye Institute that seeks to identify those mechanisms that regulate cellular growth and differentiation.
-FAU-
Tags: faculty and staff | science | medicine | research | students