Elucidating Molecular Circuits Supporting
Neuronal Signaling and Health
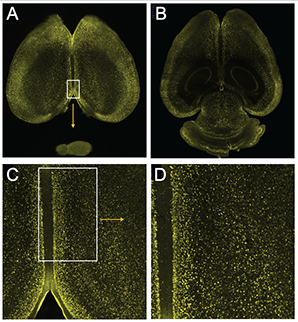
Optical sectioning of a whole mouse brain via the Blaze Light Sheet Ultramicroscope demonstrates c-fos labeling at increasing magnification moving from A to D where individual activated cells are readily detected.
c-fos labelling showing neuronal activation on a whole mouse brain. After immunolabelling and tissue clearing, brain was scanned on the Blaze Light Sheet Ultramicroscope. Credits: Lorena Areal, Paula Kurdziel and Jana Strickler.
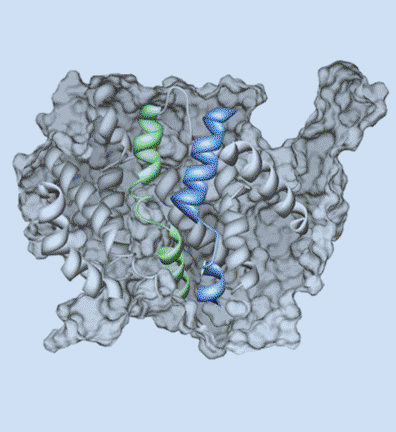
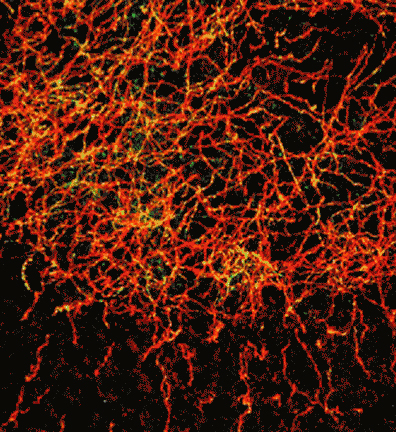
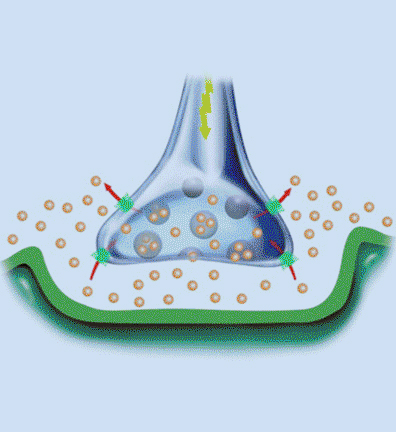
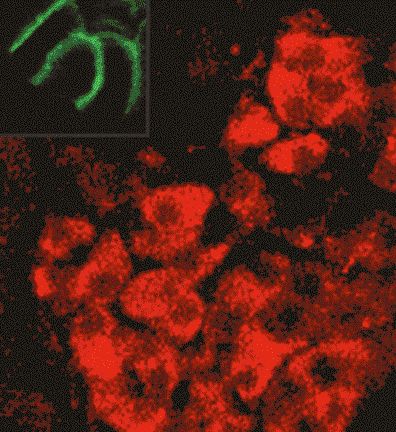
Research in the Blakely laboratory focuses on the structure, regulation and disease relevance of neurotransmitter transporters, proteins that govern the inactivation of chemical signals released at synapses to elicit target activation and inhibition and ultimately control the way we think, move and behave. The work utilizes a range of physiological, optical, genetic and genomic methods to understand how changes in transporter function can support neuroplasticity as well as neural disorders. The researchers use multiple model systems that include the powerful genetic model C. elegans, where discovery of molecules that support neural signaling and health is enhanced by rapid growth and ease of genetic manipulation. Other studies utilize genetically modified mice whose complex circuitry and behaviors align with those present in humans and can allow for modeling of neurobehavioral and neurodegenerative disorders. In collaboration, the researchers pursue studies in humans exhibiting the molecular and circuit changes they have observed in animal models and aid in the development of novel therapeutics. Together, these tools, models and paradigms are pursued to advance understanding, diagnosis and treatment of brain diseases that impact the lives of millions worldwide, disorders ranging from ADHD and autism to neuropsychiatric disorders and neurodegenerative diseases such as Alzheimer's and Parkinson's disease.



Elesclomol Diminishes Redox Imbalance in Peripheral Tissues of Mblac1 Knockout Mice.
Neghabi M, Nategh P, Stauffer AM, Hahn MK, Blakely RD, Ranji M.
J Biophotonics. 2026 Jan;19(1):e70224. doi: 10.1002/jbio.70224.
PMID: 41548934
Peripheral Inflammation Limits Serotonin Neuron Signaling Capacity via Serotonergic IL-1R1 to Reduce Neuronal Excitability and Enhance Serotonin Clearance
P. A. Gajewski, H. Iwamoto, A. N. Tillman, Z. Filliben, A. E. Walsh, N. L. Baganz, M. J. Robson, M. Zapata, N.Quan, R. D. Blakely
bioRxiv 2025.10.13.682078; doi: https://doi.org/10.1101/2025.10.13.682078
Elesclomol-Mediated Alterations of Liver Metabolism in the Context of Mouse Mblac1 Disruption
Nategh P, Neghabi M, Stauffer AM, R. D. Blakely, Ranji M.
Annu Int Conf IEEE Eng Med Biol Soc. 2025 Jul;2025:1-4. doi: 10.1109/EMBC58623.2025.11254869.
PMID: 41335708
The Ins and Outs of Dopamine Transporter Gene Manipulation: In Vivo Models of DAT Dysfunction
Stewart A, Blakely RD
Adv Neurobiol. 2025;46:235-270. doi: 10.1007/978-3-031-96364-3_10.
PMID: 41051713
Kappa opioid receptor antagonism restores phosphorylation, trafficking and behavior induced by a disease-associated dopamine transporter variant.
Mayer FP, Stewart A, Varman DR, Moritz AE, Foster JD, Owens AW, Areal LB, Gowrishankar R, Velez M, Wickham K, Phelps H, Katamish R, Rabil M, Jayanthi LD, Vaughan RA, Daws LC, Blakely RD, Ramamoorthy S.
Mol Psychiatry. 2025 May 29. doi: 10.1038/s41380-025-03055-4. Online ahead of print.
PMID: 40442453

Randy D. Blakely, Ph.D.
Executive Director
FAU Stiles-Nicholson Brain Institute
David J.S. Nicholson Distinguished Professor in Neuroscience,
Professor, Dept Biomedical Science
Charles E. Schmidt College of Medicine
Click here for Dr. Blakely’s Curriculum Vitae.
Click here for Dr. Blakely's professional biography.
Updated 1/2025