Networks
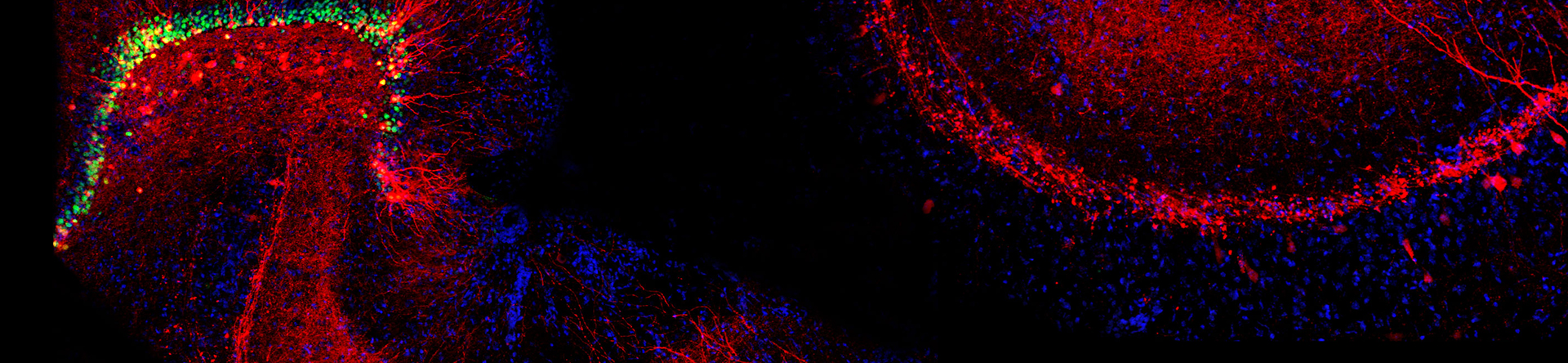
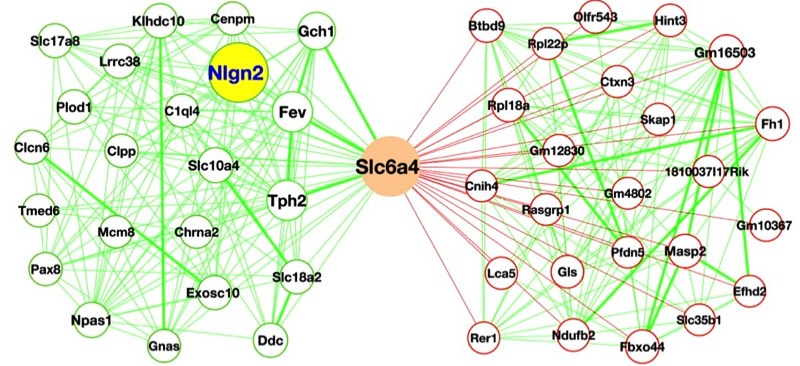
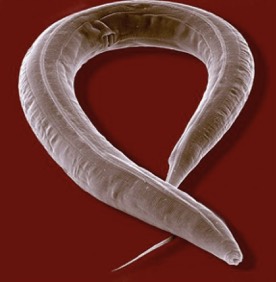 The Blakely lab continues to implement new approaches and technologies to elucidate molecular, cellular and circuit-level networks that support neuronal signaling and health. Using the powerful genetic model system Caenorhabditis elegans, and taking advantage of behaviors that emerge with loss of the dopamine transporter, we utilized unbiased genetic screens to identify and characterize genes required for the precise regulation of dopamine signaling. The genes identified in these screens have mammalian counterparts, so their identification opens up new opportunities to understand, for example, the molecular control of dopamine signaling as well as potential insights into risk for brain disorders, including Parkinson's disease, addiction, ADHD and autism. Other approaches take a top-down approach, where the complex fabric of neural circuitries is mined for the secrets that emerge when brain networks are probed in freely behaving animals. In mouse models, we utilize genetically encoded sensors for neurotransmitters and cell excitability to evaluate how fundamental behaviors derive from different neurotransmitters, to understand the impact of human disease-associated mutations, and to evaluate potential therapeutic leads in vivo. With whole-brain mapping of neuronal excitation and circuit responses to drugs and behavioral challenges, we are gaining insights into the circuit-level plasticities that afford resilience to stress during early life and how to capitalize on a broader perspective to monitor and treat the diseases of aging. Lastly, anatomically resolved single-cell transcriptome sequencing approaches are allowing us to broaden the impact of genes, proteins and drugs that ultimately govern behavior. These efforts produce high-content data streams that can be mined using AI-based approaches to more efficiently work our way through the myriad of next-generation, personalized treatments of brain disorders.
The Blakely lab continues to implement new approaches and technologies to elucidate molecular, cellular and circuit-level networks that support neuronal signaling and health. Using the powerful genetic model system Caenorhabditis elegans, and taking advantage of behaviors that emerge with loss of the dopamine transporter, we utilized unbiased genetic screens to identify and characterize genes required for the precise regulation of dopamine signaling. The genes identified in these screens have mammalian counterparts, so their identification opens up new opportunities to understand, for example, the molecular control of dopamine signaling as well as potential insights into risk for brain disorders, including Parkinson's disease, addiction, ADHD and autism. Other approaches take a top-down approach, where the complex fabric of neural circuitries is mined for the secrets that emerge when brain networks are probed in freely behaving animals. In mouse models, we utilize genetically encoded sensors for neurotransmitters and cell excitability to evaluate how fundamental behaviors derive from different neurotransmitters, to understand the impact of human disease-associated mutations, and to evaluate potential therapeutic leads in vivo. With whole-brain mapping of neuronal excitation and circuit responses to drugs and behavioral challenges, we are gaining insights into the circuit-level plasticities that afford resilience to stress during early life and how to capitalize on a broader perspective to monitor and treat the diseases of aging. Lastly, anatomically resolved single-cell transcriptome sequencing approaches are allowing us to broaden the impact of genes, proteins and drugs that ultimately govern behavior. These efforts produce high-content data streams that can be mined using AI-based approaches to more efficiently work our way through the myriad of next-generation, personalized treatments of brain disorders.